The Story of Chromium: Part 1
The Story of Chromium: Part 1
As with our earlier stories, on the creation of Steel and Nickel, we find that Chromium originates during the explosive deaths of massive stars in outer space. During this process, stars fuse hydrogen into helium and eventually into heavier elements like carbon and oxygen. This process continues until iron is formed, at which point the fusion process stops and the star collapses and eventually explodes as a supernova.
Such explosions release enormous amounts of energy, allowing for the creation of elements heavier than iron, including chromium. After chromium is formed, it can be distributed within a planet like ours, the Earth, through a process called core partitioning, where heavier elements sink towards the core during planetary formation.
On Earth, chromite which is the mineral containing chromium is heavily concentrated in a few key regions with South Africa and Kazakhstan having held the majority of identified reserves.
Many of us are first aware of this metal because of its use in Chrome plating, possibly on the first bicycle we ever owned as children. Chrome plating is a technique of electroplating a thin layer of chromium onto a metal object. A chrome plated part is said to have been chromed and, this can be decorative, providing corrosion resistance, facilitate cleaning, and increase the surface hardness. Figure 1 illustrates a chromed part of a motorcycle; these examples are all around us in our daily lives.

Chromium compounds used in electroplating are toxic and In most countries, their control and disposal is tightly regulated.
Apart from the aesthetic side of plating, Chromium is a very important strategic material needed for adding to and making several different steel types, it being the key element in making stainless steels “stainless”.
However, prior to 2007 there were no known significant deposits of chromium bearing ores in Canada. While the United States has some chromite deposits, notably in Montana, and some chromium is supplied by domestic recycling of stainless steel, the vast majority of North American chrome is imported from the aforementioned countries with large chromium reserves.
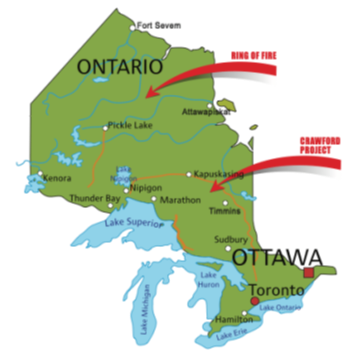
By now, everyone has heard of the “Ring of Fire” which we touched on in our earlier “Story of Nickel”. This is a vast, mineral-rich region located in the remote James Bay Lowlands of Northern Ontario. Spanning approximately 5,000 square kilometres, the area is rich in nickel, copper, platinum, gold and zinc. However, it is also one of the biggest chromium finds, one of the largest in the world. As such, The Ring of Fire is considered one of the most significant mineral deposits in Canada, with potentially great importance for the nation's economy and mining industry.
Also, north of Timmins there are other discoveries of important critical mineral deposits, particularly in the context of the Crawford Nickel project, which is also showing significant chromite deposits. It has been estimated that the Crawford project will produce 2.8 million tonnes of chromium, in excess of 6 million tonnes of nickel, 24,000 tonnes of cobalt, 490 million ounces of platinum and palladium, and many million tonnes of iron. Production is currently estimated as late 2026/early 2027.
The developing company intends to establish a new, large-scale stainless steel and alloy production facility in the Timmins region to process the raw materials from the Crawford mine. The vision is for this plant to become a large stainless steel production facility, capable of producing over 500,000 tonnes of 300-series stainless per year. A key part of the plan is to capture carbon emissions from both the nickel processing plant and the stainless-steel facility, and permanently store them in the Crawford mine's tailings. This would make the entire production process net-zero carbon, creating "green" stainless steel. Figure 2 shows the approximate locations of these chromite deposits with respect to the mining community of Timmins.
Chromite refining primarily involves converting chromite ore into ferrochromium, an alloy of iron and chromium, which is then used in the production of other steels. This process typically occurs in submerged arc furnaces, where chromite concentrate is smelted with reductants like coke and coal, along with fluxes like quartzite. The resulting ferrochromium is then further processed and used in various industrial applications, notably stainless-steel manufacturing. As a note, the global requirement for Stainless Steel has been increasing at a rate of 5-7% a year.
Traditional chromite processing can generate a lot of pollution. Green metallurgical processes are being developed to mitigate these environmental concerns. One of these is Natural Gas Reduction, a refining method using natural gas to convert chromite into a metallized chrome and iron alloy, potentially offering energy savings and reduced greenhouse gas emissions. The impact of this direct reduction processing of chromite is said to be a “game changer”, having the potential for global energy reductions equivalent to the effect of completely eliminating energy demand from a country the size of Italy. Since Canada has a plentiful amount of natural gas, this is the process of the future.
Chromium is widely used in the production of various alloys, particularly to enhance strength, hardenability, and resistance to corrosion and oxidation. It's a key ingredient in the following.
Stainless Steel:
- Chromium is essential for making stainless steel, forming a passive chrome oxide layer on the surface of the steel that protects the underlying metal from corrosion.
Low-Alloy Steels:
- Chromium is added to low-alloy steels to improve their hardenability, wear resistance, and high-temperature strength.
Nonferrous Alloys:
- Chromium is applied to nonferrous alloys, such as cobalt alloys, to improve corrosion resistance and hardness.
Superalloys:
- In high-performance superalloys used in jet engines, chromium provides resistance to high temperatures and corrosive environments.
We will expand on these other alloys that include chromium, and also where necessary discuss their weldability. This will be in Part 2 of the Story of Chromium.
Mick J Pates IWE
President PPC and Associates
Disclaimer
The information provided is intended for general interest, to educate and inform our audience. The CWB and those providing feedback to the questions do not take any responsibility for any omissions or misstatements that could lead to incorrect applications or possible solutions that industry may be facing.
How It Works content is submitted by Industry experts to the CWB Association and does not necessarily reflect the views of the CWB Group. When testing for CWB Certification or CWB Education, please refer to CWB Education textbooks or CSA standards as the official source of information.